PART 3
[The author would like to thank the geniuses behind the new Microsoft Paint for making the figures in this part possible.]
How are laser beams produced?
Laser beams are the product of stimulated emission of light. But what is stimulated emission of light? Before we can answer this question, we must first discuss how matter interacts with light.
Light absorption
Matter and light interact in two ways: matter can absorb light or it can emit light. When an atom absorbs a photon of light (a photon of light is a quantum of light energy), it takes the energy of that photon and one of its electrons become excited (although not sexually). An electron orbits the nucleus of an atom in one of the possible orbits. Larger orbits (orbits that take the electron farther from the atom) have greater energy. Normally, an electron will orbit the nucleus in the smallest possible orbit. This orbit is called the ground state. When an electron gets excited, it jumps from the ground state to an orbit with a higher energy. Such an orbit is called an “excited state”. An electron is excited when it is on one of the excited states. The excitation of an electron (represented by the symbol e–) by a photon is illustrated by Figure 1 below.
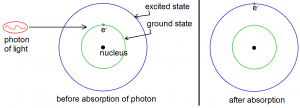
The ground state and one excited state shown in Figure 1 are just two of the many possible orbits of an electron around the nucleus. From quantum mechanics we know that the possible orbits are quantized. The quantization of electron orbits is important, so let us stray a bit from the main topic to discuss it.
Using the most important equation of quantum mechanics, Schrodinger’s equation, physicist were able to discover that in a given atom, the electron can only have a certain set of energies. This set of energies is called the spectrum of the atom. The possible energies are denoted by E0, E1, E2, E3 and so on. E0 is the smallest energy that an electron can possibly have. This is called the ground state energy. Obviously, this is the energy of an electron when it is in the ground state. (Recall that ground state is the smallest possible orbit.) E1 is the energy of the first excited state, E2 the energy of the second excited state, and so on. The energy of a higher excited state is always greater than the energy of the excited states before it. In other words, E0 is less than E1, which in turn is less than E2, which again in turn is less than E3 and so on. (E0 < E1 < E2 < E3 < …) Now, here’s the most important bit. An electron in an atom cannot have energy between E0 and E1. It is simply impossible for an electron to have energy greater than E0 but less than E1. Also, an electron can never have energy between E1 and E2, or between E2 and E3, and so on. The set {E0, E1, E2, E3, …} gives the only possible energies of the electron.
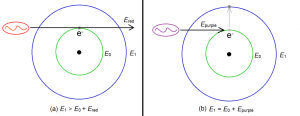
Now we go back to absorption. Consider an electron initially in the ground state, so that its energy is initially E0. Suppose then that a photon of light having energy Ephoton hits the electron. This photon will be absorbed by the atom and its energy will then be added to the original energy of the electron. The new energy of the electron after absorbing the photon will therefore be:
Enew = E0 + Ephoton.
But take note of this very important fact: Enew must be one of the allowed energies of the electron. That is, Enew must be one of the following: E1, E2, E3 and so on. This means that if the energy of the photon, Ephoton, is such that Enew is not one of the allowed energies of the electron, then the photon will be totally ignored – the atom will not absorb it. An atom will absorb a photon only if it will make Enew be one of the allowed energies of the electron.
Figure 2 below illustrates a particular example. The red photon is ignored by the electron because its energy is not enough to make the electron jump to the first energy level. The purple photon, however, was absorbed, because its energy is just right to make the electron jump from the ground state (where energy is E0) to the first excited state (where energy is E1). In other words, the sum of the red photon’s energy and the ground state energy (Ered + E0) is not equal an allowed energy level, so electron ignores the photon completely. On the other hand, the sum of the energy of the purple photon and the ground state energy (Epurple + E0) is exactly equal to E1. Since E1 is an allowed energy, the electron will absorb the purple photon.
Spontaneous Emission
The emission of light is just the inverse process of the absorption of light. Here, an electron in a certain energy level (that is, a certain excited state) falls down to a lower energy level (a lower excited state or the ground state). When an electron falls down to a lower energy level, we say that it “relaxes”.
Relaxation is just the inverse process of excitation. (Although this is only true for electrons. In humans, it’s certainly not the case.) When an electron relaxes, it loses energy. The energy lost is of course equal to the difference between the original energy and the new energy. This lost energy becomes the energy of an emitted photon. For example, consider an electron initially in the first excited state. This means that its initial energy is E1. Suppose that this electron relaxes to the ground state, so that its final energy is E0. It is not difficult to see that this electron lost energy, and that the lost energy amounts to E1 – E0. This energy becomes the energy of the emitted photon.
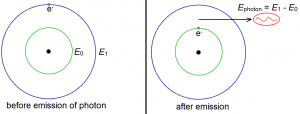
Of course, an electron does not have to come from the first excited state. It may come from the second excited state, or form the third excited state, and so on. The higher the electron falls, the greater the energy of the photon it emits.
The situation shown on Figure 3, however, is just one type of emission called spontaneous emission (which does not have to be nocturnal). This is called so because the atom spontaneously emits a photon of light. Most light sources we know produce light by spontaneous emission. In a fluorescent light bulb, for example, electricity is used to excite the electrons of the atoms of a gas (usually the gas is mercury vapor). But the excitations are only momentary, because the electrons spontaneously jump back to the ground state and in the process emit photons of light. (These photons of light, however, are not what we “see” when we look at a fluorescent lamp. The photons produced in the said process have wavelengths in the ultraviolet region. This means that they are invisible to the human eyes. These invisible ultraviolet photons, however, cause the phosphorescent compound coating the inner lining of the lamp to glow. The phosphorescent compound is the white powder that is found inside the bulb.)
Laser beams, however, are not produced by spontaneous emission of light but rather by stimulated emission of light, hence the phrase “stimulated emission of radiation”. The very suggestive and juicy term stimulated emission describes the phenomenon where an atom emits light due to the stimulation of another photon. Basically, stimulated emission is just like spontaneous emission, only that it is not spontaneous.
Why Stimulated Emission is Amazing
Stimulated emission has the following remarkable property: when a photon stimulates an atom to emit another photon of light, the emitted photon will have the same wavelength, phase, polarization and direction as the original photon. In short, the emitted photon is just a Doppelganger of the photon that stimulated its emission!
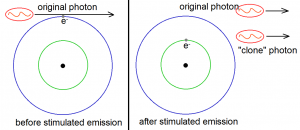
We can now see that when one has enough atoms with electrons in the excited states, then one can use stimulated emission to greatly increase the intensity of a certain light. This is because light intensity can also be measured through the number of photons in a given beam. The more photons there are, the greater the intensity. But notice that stimulated emission has the ability to practically double the number of photons of the given light source. This is illustrated in Figure 5 below, where three atoms with excited electrons are used to double the number of photons from three to six. This is where the “light amplification” part of LASER comes from. (Recall that laser means “light amplification via stimulated emission of radiation”.)
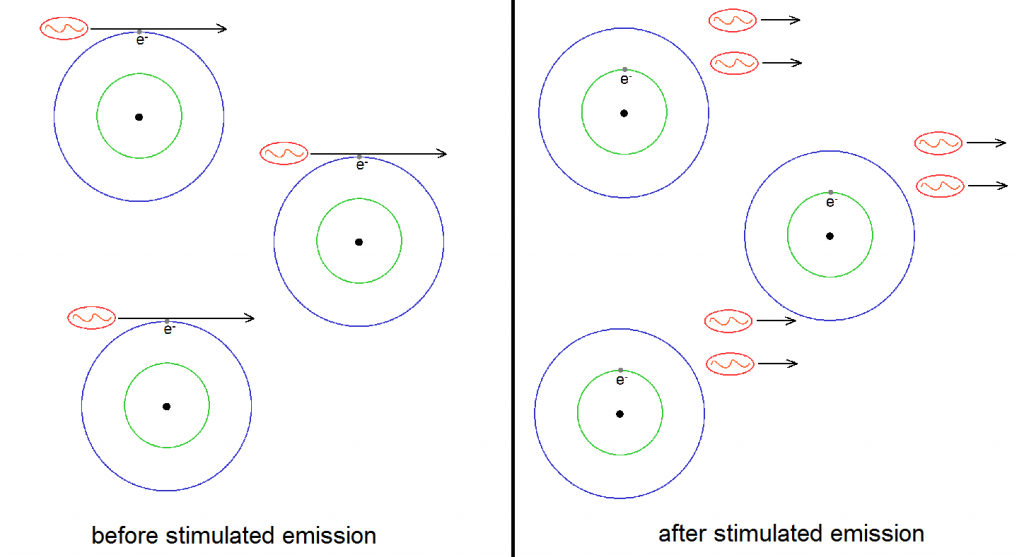
But the light is not merely amplified by stimulated emission. The beam produced due to stimulated emission has the remarkable property of being composed to photons with the same wavelength (and therefore color), phase, polarization and direction. This is the reason why laser beams are highly monochromatic and coherent. Remember that to be monochromatic means to be composed of only one color. Since a laser is practically composed of an army of photon clones, a laser beam is very monochromatic indeed. Also, since the emitted photons have the same phase (which can be thought of as the tempo or vibration rhythm of the photon), polarization and direction, a laser beam has very high spatial and temporal coherence. In short, all the remarkable properties of laser beams are derived from a very remarkable property of stimulated emission!
Laser Devices
But now I hear the result-oriented and practically-minded among you say: “But how do we prepare a collection of atoms with excited electrons? Does this not mean that we also need photons to excite these electrons? And does this not mean that we are cancelling the amplifying effect of stimulated emission because we will be using photons to double the number of photons? Isn’t it like investing 500 pesos to gain 500 pesos?” Well, the answer is that light absorption is not the only way to excite electrons. There are many other ways to cause electrons to become excited, like hitting them with electricity or heating to very high temperatures them. There are many different kinds of lasers and each kind uses a different strategy to prepare atoms with excited electrons. But once such atoms are prepared, stimulated emission can then be exploited to double the number of photons from a certain light source, thereby increasing the intensity of the light.
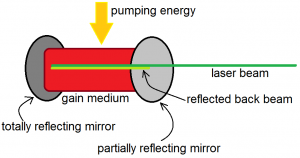
In fact, in laser devices, the number of photons is not merely doubled, but is increased by several orders of magnitude. This is done by placing the gain medium in an optical resonator, as shown in Figure 6 below. The gain medium is the material that is used to amplify light. An optical resonator is a combination of two mirrors that make light bounce back and forth between them. One of these mirrors is a totally reflective mirror, while the other is a partially reflective and mirror. The partially reflective mirror allows some of the laser beam to pass while it reflects the rest. The beam that is reflected back can again stimulate even more atoms with excited electrons to emit photons. When it reaches the other end, it will be reflected by the totally reflecting mirror, and will then pass through the gain medium again, before reaching the partially reflecting mirror, where the cycle begins again. Notice, however, that once an atom is stimulated to emit radiation, its electron has already relaxed to the ground state. This means that after several rounds of stimulated emission, all the atoms will have ground state electrons only. So that the production of the beam is continuous, a “pumping energy” must be supplied to the gain medium constantly so that the atoms of the gain medium always have excited electrons.
Summary
Now let us sum up all that we have discussed in this series. We started this series with a question: What in the world is laser? Well, laser is short for Light Amplification via Stimulated Emission of Radiation. A laser device is a device that produces laser beams via a phenomenon known as stimulated emission. Because of the remarkable properties of stimulated emission, laser beams have the extraordinary property of being monochromatic and coherent. By saying that it is monochromatic, we mean that it only has one wavelength. By saying that it is coherent, we are saying that its photons are oscillating or vibrating “to the same rhythm and beat”.
Now I hear the astute ones among you say, “Wait a minute, I see an inconsistency here. One minute you describe light as being made up of waves; electromagnetic waves, to be precise. But now you are describing them using particle-like entities called photons. What really is light made of, electromagnetic waves or photons?” This is a very good question, and it has a very remarkable answer: Light is made both of electromagnetic waves and photons. But how is that possible?
Now that is a topic for a separate series.


if I may suggest to make this series a bit more accessible to non-physicist readers like me, maybe you can expound a bit more on laser applications for consumer goods like laser pointers and mice. I'd always been wondering how these things work and how a laser could be generated from such relatively low-powered devices. Are they "real" lasers from a technical perspective? because you mentioned that lasers can be generated via different methods so how does a layman know if its actually a laser or just a thinly focused beam of light (like say, focused through a lens)?
A laser is parallel; it is not refracting or colliding light.
I am an expert, i am a phyisicist, i am not trying to impress but to educate. And, everything i mentioned is from my stock knowledge and experience, although they are all as you mentioned "wikiable". I noted that you missed out on something very important. There are ways to simplify the concept of population inversion and i was expecting you would. Have you worked with lasers before?
No one is questioning your expertise. And no one was questioning your knowledge either.
My problem with the concept of population inversion was not that I could not simplify it. It was that discussing it would lead me deep into statistical territory, and I found that including it would make the article too long for my taste.
No, I have not worked with lasers before. Well, perhaps a bit, during my undergrad. Some simple experiments with He-Ne and CO2 lasers. My undergrad research work was purely theoretical.
I am just on my way to grad school. I intend to specialize on the statistical aspects of astrophysics or turbulence, although the final decision rests on my thesis adviser.
Do you happen to write popular science articles as well? If so, can you please lead me to a link were I can read your articles? Much appreciated.
No problem mate! Good luck with grad school! =) I write papers for scientific journals, and do reviews every now and then. I think you are more competent to write articles that would be more palatable to a layman. i see potenttial there, so keep it up. I'll be available if you have any questions or clarifications, especially in the field of optics. Cheers!
Again, thanks for your comments. Keep 'em coming.
Don't worry, I'll write and post more science articles here and you are very welcome to give a critique and comment to every one of 'em.
I disagree with the generalization that the energy of an incident photon will excite the electron to an equal energy level. That is an idealization and it does not occur in nature. What really happens is that the incident photon has an energy higher than the energy difference between the excited state that an electron will be excited to, and the ground state. Thus, the electron is excited to an excited energy level that is less than the energy of the incident photon (unless we are talking about two-photon, or multiphoton excitation processes, which is beyond the scope of this discussion). It is for this reason that fluorescence occurs, wherein a short wavelength of light (ultraviolet light for example) strikes a material, exciting its atoms, molecules or electrons, causing it to emit light of a longer wavelength (visible light for example). You can try this by shining a UV lamp (similar to that used to inspect for fake bills) onto a smooth sheet of coupon bond and you would notice that the surface of the sheet of paper exposed to the light emits blue light. UV is invisible, whereas blue is visble. Now back to the discussion on lasers, a very important condition to produce a laser (which i think was not discussed here) is to have "population inversion". Atoms, molecules, electrons, are normally in their ground state and population inversion simply means that you have a significantly greater number of these atoms, molecules, electrons, in their excited state, than in their ground state. This condition leads to simultaneous, radiative de-excitation (going back to the ground state) of the atoms, molecules, electrons and their emission of photons of the same wavelength (color) and frequency (which is simply the inverse of wavelength). Having an optical resonator simply enhances the amplitude of the photons (think of light as a wave) as they bounce back and forth in the chamber and interact with other photons of the same frequency that are emitted as a result of population inversion and subsequent radiative de-excitation. The amplitudes of these photons add up or get "amplified" because they have the same phase (think of waves in the beach that move at the same speed and meet at one point, this is also the mechanism behind tsunamis). In other words, the waves interfere constructively, as opposed to destructively, with each other. Now the poster focused on light causing laser excitation, and examples of these types of lasers are some solid state lasers such as the Nd:YAG, ruby and dye lasers (these are not solid state). Nd:YAG and ruby lasers use UV flashlamps as the excitation source, and emit light in the infared (Nd:YAG) and visible (ruby laser), while dye lasers can emit light from the visible to infrared range depending on the type of excitation laser and the dye used. Other laser are excited with the application of a voltage potential like gas lasers (CO2, He-Ne, Argon ion) and with current (diode lasers for laser pointers).
Everything you say is valid and well taken.
However, take note that I am writing for a general audience and not for a panel of experts or a group of specialist. Heck, I did not even write for science majors! The audience I had in mind included history, literature, philosophy and fine arts majors. I even had in mind non-majors and high school drop outs! Science writers always use idealizations in order to keep it simple and to make the general reader understand the very gist of the physics.
For example, have you read any Hawking or Brian Greene book? If you're an expert, you would find many simplifications and idealization in their popular books. But such simplifications are necessary. You cannot throw all the subtleties of a subject to the non-specialist. Would you read a popular biology book that is full of technicalities? Or would it be a popular biology book in the first place?
Always remember, when you are writing to popularize science, you are writing to EXPRESS, not to IMPRESS. In short, your main goal is not to exhibit to the world how smart you are; rather, your goal is to make to make the non-specialist understand the basics of a subject.
By the way, everything you said in your comment are Googleable or Wikiable. Today, everything anyone needs to know can be found on the internet. My goal here is to add my own personal take on the subject. and of course, to popularize it so that non-specialists can appreciate it.
Although I have to admit, it was a painful decision for me to edit out the population inversion part. Would you like me to put it back? Do you think it would make the article more illuminating for the non-specialist?
Anyways, thank you very much for your (very technical) comment.